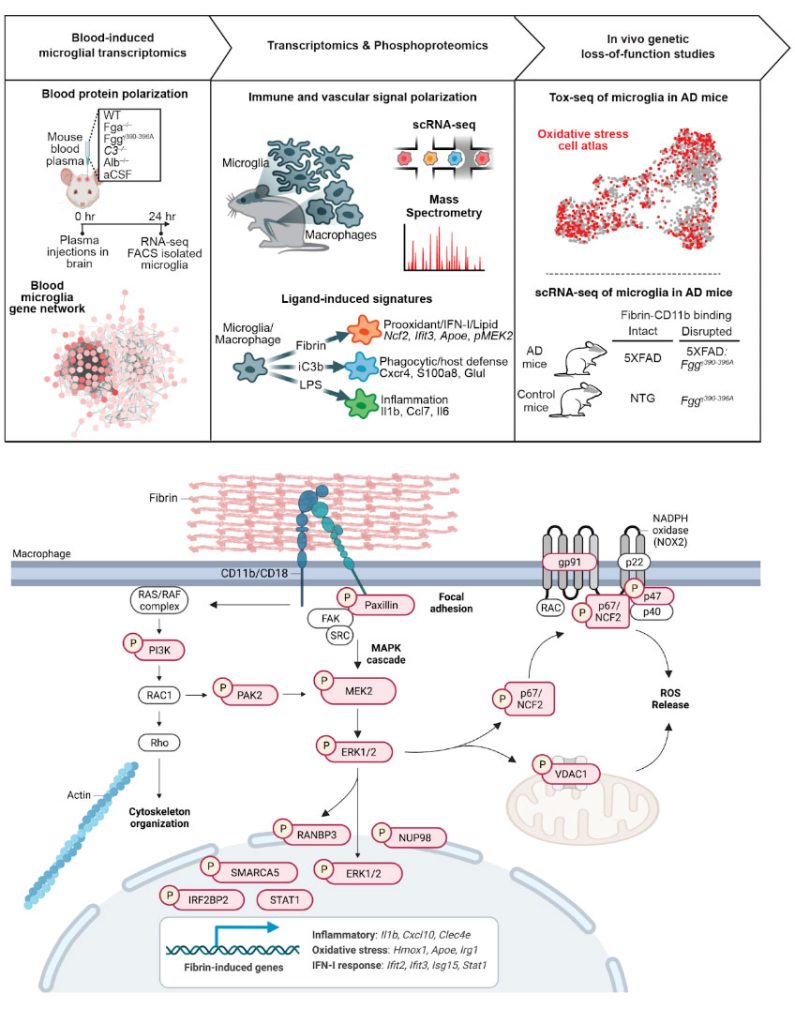
How are neurotoxic microglia transcriptionally regulated in AD?
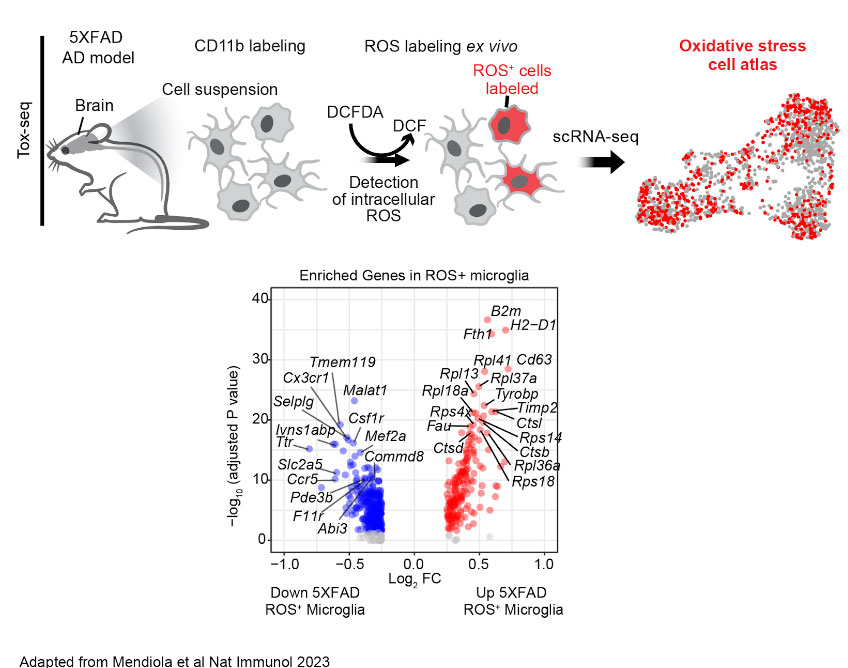
Oxidative stress is inversely coupled to brain function and neuropathology in AD. However, the mechanisms of microglia and other cells in the brain that mediate oxidative stress are largely unknown. Using Tox-seq, we recently generated the first molecular map of the oxidative stress transcriptome of microglia in neurodegeneration. We discovered that oxidative stress-producing microglia are transcriptionally distinct from cells that do not produce oxidative stress in the 5XFAD mouse model of AD. While we know ROS+ microglia converge to a core oxidative stress gene signature, it remains unknown the mechanisms that regulate oxidative stress genes and their functions in AD. We now investigate the oxidative stress epigenome to understand how neurotoxic microglia are transcriptional regulated in mouse models of AD.
By targeting cis-regulatory regions of oxidative stress-producing microglia, we open a novel therapeutic strategy for AD in which the early and progressive tissue damage caused oxidative stress are prevented. This work has the potential to open therapeutic avenues for AD and related dementias to prevent neurodegeneration that are not currently addressed by other treatments.